NDA Group replaced ISDN with Teams Calling via AMVIA: 30% cost savings, 2-month deployment, global expert support enabling hybrid working and pharmaceutical regulatory agility.
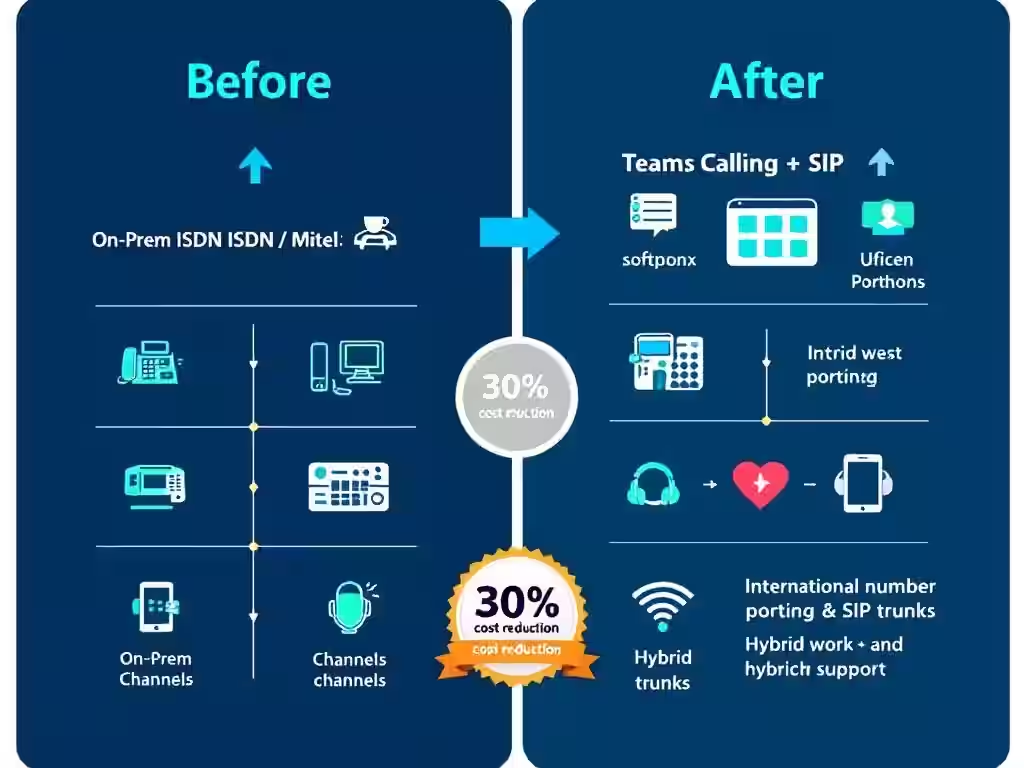
The challenge: International pharmaceutical consultancy NDA Group needed to replace aging ISDN systems to support hybrid working across four European offices. Legacy infrastructure (£12.75/channel monthly) was inflexible and didn't support remote regulatory work. The solution: Microsoft Teams Calling via AMVIA's human-first model replaced ISDN in 2-month deployment, reducing costs to £4.95/trunk monthly (30% savings), enabling true device flexibility, and providing direct expert support across time zones. Result: operational agility supporting pharmaceutical regulatory excellence.
NDA Group operates as a leading regulatory drug and device development consultancy across four international offices. Their business requires rapid expert response to regulatory challenges, complex multi-jurisdictional submissions, and seamless client collaboration. In 2024, this mission ran into a critical constraint: aging telecommunications infrastructure.
Their existing Mitel solution running on ISDN lines was built for on-site, phone-centric operations. But pharmaceutical consulting has transformed. Regulatory experts now work from client sites, home offices, and sometimes across multiple countries simultaneously. Fixed-line phone systems designed for static office locations became operational friction.
The problem was immediate and measurable: Consultants couldn't work effectively from home. Client calls required them to return to office. Remote teams in different time zones had no unified communication platform. The infrastructure that once enabled operations now constrained them.
Most critically, as businesses shifted to hybrid models post-pandemic, studies showed 73% of employees require compelling reasons to return full-time to offices. For NDA Group, their communication systems worked against recruitment and retention—not for it.
NDA Group investigated standard telecom providers. Most offered standard solutions: migrate to their cloud system, pay setup fees, train teams on new interfaces. The common pattern: one-size-fits-all deployments with minimal local expertise across four different countries.
International deployments created additional complexity. Different regulatory frameworks, different local ISP requirements, different time zones for support access. Large providers typically route international support through centralized call centers—not ideal when you need immediate expert access to solve cross-border connectivity issues.
NDA Group needed something different: a partner who understood their international complexity, could coordinate across multiple territories, and provided direct expert access when regulatory deadlines created urgent needs.
AMVIA's positioning as a human-first connectivity alternative directly addressed NDA Group's frustrations with impersonal provider models. Rather than routing support through automated systems, AMVIA's no-voicemail policy (0333 733 8050) guarantees direct access to technical experts.
For pharmaceutical consultants managing confidential regulatory submissions, this direct relationship proved immediately valuable. When urgent technical issues arose, they could speak directly with someone who understood their requirements—not navigate multi-tier support structures.
AMVIA's independence—maintaining relationships with 50+ network suppliers rather than being locked to single-provider infrastructure—meant optimal service delivery across all four of NDA Group's office locations. What works best in Sweden might differ from Germany or France. AMVIA's approach: choose optimal infrastructure for each territory.
NDA Group teams already used Microsoft Teams for video meetings and collaboration. The decision to deploy Teams Calling wasn't revolutionary—it was natural. One platform. Familiar interface. Minimal training required.
But the deeper value emerged when integrated with Microsoft 365 cloud productivity tools. Within Teams, consultants could:
For pharmaceutical work where regulatory timelines are tight and expert coordination is constant, this unified platform eliminated friction that the old phone-centric model created.
International number porting across multiple jurisdictions is technically complex. AMVIA handled this through detailed project management: pre-deployment stakeholder engagement at each office, customized training materials adapted to local workflows, and phased rollout preventing disruption during peak regulatory periods.
Timeline: Two months from initial planning to full deployment across all four offices. This measured pace prioritized business continuity over aggressive go-live dates—critical when regulatory submission deadlines are non-negotiable.
The change management piece proved equally important as technical execution. Pharmaceutical consultants are precise, deadline-focused professionals. AMVIA worked with local teams to understand how communication tools fit into their regulatory workflows—not just how to use them technically.
Result: All offices moved to Teams Calling on scheduled dates without service disruption or regulatory impact.
Legacy ISDN channels: £12.75 monthly per line. Teams Calling SIP trunks through AMVIA: approximately £4.95 monthly per trunk. For international pharmaceutical consultancy with multiple concurrent calls across time zones, this isn't trivial savings.
Annual cost reduction: Roughly 30% savings on voice infrastructure costs. For a team spread across multiple countries, this translates into significant budget recovery—budget that NDA Group could redeploy toward core regulatory expertise and talent.
But the value extended beyond cost reduction. The old ISDN system required desktop phones at every workstation. The new model enabled almost complete removal of physical phones—consultants used Teams on laptops and mobile devices. This flexibility meant working from anywhere without compromising communication capability.
The immediate benefits became apparent within weeks:
Pharmaceutical regulatory work doesn't follow 9-to-5 schedules. Regulatory authority questions arrive unexpectedly. Clients need expert access immediately. With Teams Calling and AMVIA's 24/7 direct expert support across time zones, response times improved. Critical issues got resolved without multi-hour delays navigating support queues.
The old system required centralization—all phone management routed through Swedish IT headquarters. Teams Calling enabled each office to manage their own communications locally. This reduced dependency on central IT while improving responsiveness to local issues. Faster problem-solving at the office level.
Regulatory consultants travel frequently. Client site visits, regulatory meetings, conferences. With Teams Calling, they could maintain full communication access regardless of location. The device became flexible—laptop, tablet, mobile phone—all ran the same unified communications experience.
For businesses trying to attract and retain expertise in competitive fields, this flexibility became a genuine employment advantage.
In pharmaceutical consulting, client perception matters. When NDA Group consultants can seamlessly move between calls, document editing, and video collaboration—all within integrated platform—it signals capability and professionalism.
More importantly, it enables faster, more responsive service delivery. Regulatory submissions often involve rapid back-and-forth with regulatory authorities. Unified communications enabled by modern infrastructure compressed decision cycles.
For competitive pharmaceutical consulting markets where technical capability influences client selection, this infrastructure advantage became strategic positioning.
Communications don't exist in isolation. NDA Group's modern infrastructure integrates with complementary services:
Business broadband foundation: Reliable office broadband provides stable, high-bandwidth connectivity for all four office locations—foundation supporting cloud-based communications.
Cybersecurity oversight: Managed cybersecurity services protect pharmaceutical consultancy data—regulatory submissions, client confidential information, regulatory correspondence all classified and protected.
SD-WAN optimization: SD-WAN solutions across multiple international locations optimize traffic routing, ensuring consistent performance regardless of geographic distribution.
AMVIA's independent provider status enabled coordination across these services—optimized as integrated strategy rather than disconnected vendor relationships.
The pharmaceutical industry increasingly recognizes that modern communications infrastructure supports strategic business outcomes. Recent industry data shows over 85% of biopharma executives investing in digital transformation—including communication infrastructure—to build operational resilience.
But NDA Group's experience reveals that technology alone doesn't drive transformation. The software (Microsoft Teams) was good, but success required human expertise in deployment, ongoing direct expert access when issues arose, and partnership approach prioritizing business continuity over aggressive timelines.
Large providers often default to fastest possible deployments—not always best for business-critical operations. AMVIA's measured two-month rollout prioritized zero disruption to regulatory work. That partnership mindset made the difference.
Pharmaceutical work continues evolving toward digital-first processes. AMVIA's relationships with multiple technology vendors provide early access to emerging capabilities: 5G integration, advanced collaboration tools, security enhancements.
As regulatory requirements tighten and confidentiality demands increase, NDA Group gains advantage through proactive connectivity partner. Rather than reacting to new requirements, they're positioned to anticipate them.
NDA Group achieved tangible business outcomes through this transformation:
But the intangible benefits proved equally important: employees gained flexibility, consultants could work more effectively, operational agility improved, and the organization signaled technological sophistication to clients evaluating regulatory consultants.
In increasingly competitive pharmaceutical markets, these advantages compound into genuine competitive differentiation.
If you're managing pharmaceutical operations where regulatory timelines are critical, consultants need flexibility, or international coordination is constant, evaluate whether your current communication infrastructure supports these requirements.
NDA Group's transformation serves as template: identify the gap between current infrastructure capabilities and business requirements, select platforms aligned with existing technology (Teams if your organization already uses Microsoft 365), partner with human-first providers prioritizing your business continuity, and approach deployment as strategic initiative—not just technology refresh.
The pharmaceutical industry's increasing digital sophistication creates competitive pressure for modern communications infrastructure. Organizations like NDA Group that move decisively gain advantage. Those delaying find themselves increasingly disadvantaged competing with rivals offering superior responsiveness and capability.
Ready to evaluate modern communications for your pharmaceutical organization? Contact AMVIA specialists: 0333 733 8050 (direct to experts, no voicemail) or request consultation. We analyze your current infrastructure, identify transformation opportunities, and deliver implementation strategies prioritizing your business continuity and operational excellence.
Monthly expert-curated updates empower you to protect your business with actionable cybersecurity insights, the latest threat data, and proven defences—trusted by UK IT leaders for reliability and clarity.
